Understanding the Electron Configuration: AR 4s虏 3d鹿鈦?4p鈦?Represents Which Element
When you encounter the electron configuration AR 4s虏 3d鹿鈦?4p鈦? you are looking at the distribution of electrons in an atom’s energy levels. This configuration is a key to identifying the element in question. Let’s delve into the details of this configuration and the element it represents.
Breaking Down the Electron Configuration
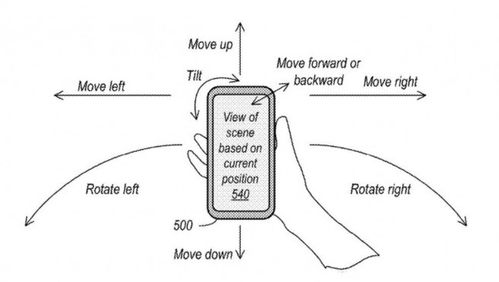
The electron configuration AR 4s虏 3d鹿鈦?4p鈦?can be broken down into its individual components:
| Shell | Subshell | Electrons |
|---|---|---|
| 4s | 4s | 2 |
| 3d | 3d | 10 |
| 4p | 4p | 6 |
This table shows that the element has a total of 18 electrons. The electron configuration follows the Aufbau principle, which states that electrons fill the lowest energy orbitals first before moving to higher energy levels.
Identifying the Element
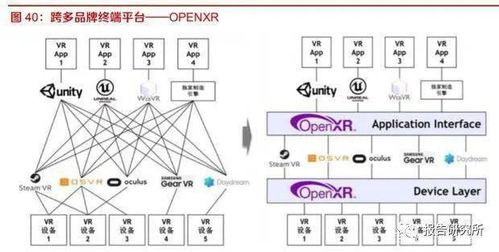
With the electron configuration AR 4s虏 3d鹿鈦?4p鈦? we can determine the element by counting the total number of electrons. In this case, there are 18 electrons. To find the element, we need to look at the periodic table and find the element with an atomic number of 18.
Upon examining the periodic table, we find that the element with an atomic number of 18 is Argon (Ar). Argon is a noble gas, which is a group of elements known for their stability and lack of reactivity. Noble gases have a full outer electron shell, which makes them very stable.
Properties of Argon

Argon is a colorless, odorless, and tasteless gas at room temperature and pressure. It is the third most abundant gas in the Earth’s atmosphere, after nitrogen and oxygen. Here are some key properties of Argon:
| Property | Description |
|---|---|
| Atomic Number | 18 |
| Atomic Mass | 39.948 |
| Boiling Point | -185.8掳C |
| Melting Point | -189.2掳C |
| Electron Configuration | 1s虏 2s虏 2p鈦?3s虏 3p鈦?4s虏 3d鹿鈦?4p鈦?/td> |
Argon is used in various applications due to its inert nature. It is commonly used as a protective atmosphere in welding and as a coolant in cryogenics. Additionally, Argon is used in the production of light bulbs and fluorescent tubes, as it does not react with the filament or glass.
Conclusion
Understanding the electron configuration AR 4s虏 3d鹿鈦?4p鈦?allows us to identify the element as Argon. This noble gas has unique properties and is widely used in various applications. By examining the electron configuration, we can gain insights into the element’s atomic structure and its behavior in different environments.






