Molar Mass of Argon: A Comprehensive Overview
The molar mass of Argon, a colorless, odorless, and tasteless noble gas, is a fundamental property that defines its chemical behavior and physical characteristics. In this article, we delve into the various aspects of the molar mass of Argon, exploring its significance, determination methods, and applications.
What is Molar Mass?
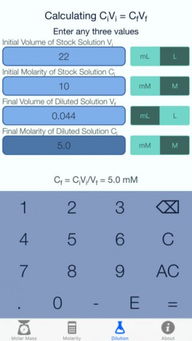
Molar mass is the mass of one mole of a substance, expressed in grams per mole (g/mol). It is calculated by summing the atomic masses of all the atoms in a molecule or compound. For elements like Argon, which exist as single atoms, the molar mass is simply the atomic mass of the element.
Atomic Mass of Argon
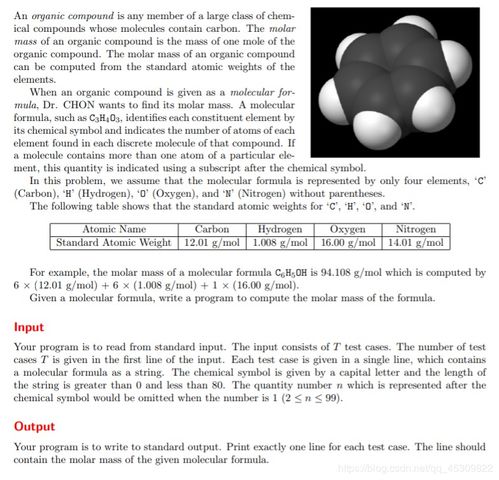
The atomic mass of Argon is approximately 39.948 g/mol. This value is derived from the weighted average of the isotopic masses of Argon, taking into account their natural abundance. The most abundant isotope of Argon is Argon-40, with an atomic mass of 40.948 g/mol, accounting for about 99.6% of all Argon atoms.
Calculating Molar Mass
Calculating the molar mass of Argon is straightforward. Since Argon is a monatomic element, its molar mass is equal to its atomic mass. Therefore, the molar mass of Argon is 39.948 g/mol.
However, if you were to calculate the molar mass of a compound containing Argon, you would need to consider the atomic masses of all the elements in the compound and their respective stoichiometric coefficients. For example, the molar mass of Argon chloride (ArCl) would be calculated as follows:
| Element | Atomic Mass (g/mol) | Stoichiometric Coefficient | Contribution to Molar Mass |
|---|---|---|---|
| Argon | 39.948 | 1 | 39.948 |
| Chlorine | 35.453 | 1 | 35.453 |
| Total | 75.401 |
As you can see, the molar mass of Argon chloride is 75.401 g/mol.
Significance of Molar Mass
The molar mass of Argon has several important implications:
-
It helps in determining the density of Argon, which is crucial for various applications, such as in balloons and airships.
-
It is essential for calculating the amount of substance in a given mass, which is vital for chemical reactions and stoichiometry.
-
It aids in identifying and characterizing Argon, as different isotopes have distinct molar masses.
Determination of Molar Mass
The molar mass of Argon can be determined using various methods, including:
-
Mass Spectrometry: This technique measures the mass-to-charge ratio of ions, allowing for the determination of the molar mass of Argon isotopes.
-
Gas Chromatography: By separating Argon from other gases and measuring its retention time, the molar mass can be calculated.
-
Thermal Conductivity: This method involves measuring the thermal conductivity of Argon, which is related to its molar mass.
Applications of Molar Mass
The molar mass of Argon has numerous applications across various fields:
-
Medical Field: Argon is used in cryosurgery, where its low boiling point allows for the freezing of tissues.
-
Food Industry: Argon is used to displace oxygen in packaging, extending the shelf life of food products.
-
Manufacturing: Argon is used as a protective atmosphere in







